?What is Electricity
Power is surrounding us- - driving innovation like our mobile phones, PCs, lights, fastening irons, and climate control systems. It's hard to escape it in our advanced world. Notwithstanding when you attempt to escape power, it's as yet at work all through nature, from the lightning in a tempest to the neural connections inside our body. Yet, what precisely is power? This is a confounded inquiry, and as you burrow further and make more inquiries, there truly is certainly not a conclusive answer, just theoretical portrayals of how power communicates with our environment.
Power is a characteristic wonder that happens all through nature and takes a wide range of structures. In this instructional exercise we'll concentrate on flow power: the stuff that controls our electronic contraptions. We will probably see how power streams from a power source through wires, illuminating LEDs, turning engines, and fueling our specialized gadgets.
Power is quickly characterized as the stream of electric charge, yet there's such a great amount behind that basic explanation. Where do the charges originate from? How would we move them? Where do they move to? How does an electric charge cause mechanical movement or make things light up? Such huge numbers of inquiries! To start to clarify what power is we have to zoom route in, past the issue and particles, to the molecules that make up all that we associate with throughout everyday life.
This instructional exercise expands on some essential comprehension of material science, compel, vitality, particles, and [fields](http://en.wikipedia.org/wiki/Field_(physics)) specifically. We'll disregard the nuts and bolts of every one of those material science ideas, however it might counsel different sources also.
Going Atomic
To comprehend the essentials of power, we have to start by concentrating in on molecules, one of the fundamental building squares of life and matter. Iotas exist in over a hundred unique structures as substance components like hydrogen, carbon, oxygen, and copper. Iotas of numerous kinds can consolidate to make particles, which fabricate the issue we can physically observe and contact.
Iotas are modest, extending at a maximum to around 300 picometers in length (that is 3x10-10 or 0.0000000003 meters). A copper penny (on the off chance that it really were made of 100% copper) would have 3.2x1022 molecules (32,000,000,000,000,000,000,000 iotas) of copper inside it.
Indeed, even the iota isn't little enough to clarify the functions of power. We have to jump down one increasingly level and look in on the building squares of molecules: protons, neutrons, and electrons.
Building Blocks of Atoms
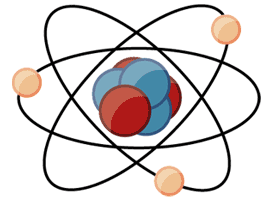
A molecule is worked with a mix of three particular particles: electrons, protons, and neutrons. Every molecule has a middle core, where the protons and neutrons are thickly stuffed together. Encompassing the core are a gathering of circling electrons.
An exceptionally basic particle display. It's not to scale but rather accommodating for seeing how a particle is assembled. A center core of protons and neutrons is encompassed by circling electrons.
Each molecule must have no less than one proton in it. The quantity of protons in a particle is essential, since it characterizes what compound component the iota speaks to. For instance, a molecule with only one proton is hydrogen, an iota with 29 protons is copper, and a particle with 94 protons is plutonium. This check of protons is known as the iota's nuclear number.
The proton's core accomplice, neutrons, fill a critical need; they keep the protons in the core and decide the isotope of an iota. They're not basic to our comprehension of power, so we should not stress over them for this instructional exercise.
Electrons are basic to the operations of power (see a typical subject in their names?) In its most steady, adjusted express, a molecule will have indistinguishable number of electrons from protons. As in the Bohr particle display beneath, a core with 29 protons (making it a copper iota) is encompassed by an equivalent number of electrons.
As our comprehension of particles has advanced, so too has our strategy for demonstrating them. The Bohr demonstrate is an extremely helpful molecule display as we investigate power.
The particle's electrons aren't all eternity bound to the iota. The electrons on the external circle of the molecule are called valence electrons. With enough outside power, a valence electron can escape circle of the iota and turn out to be free. Free electrons enable us to move charge, which is the thing that power is about. Talking about charge...
Streaming Charges
As we referenced toward the start of this instructional exercise, power is characterized as the stream of electric charge. Charge is a property of issue - simply like mass, volume, or thickness. It is quantifiable. Similarly as you can evaluate how much mass something has, you can quantify how much charge it has. The key idea with charge is that it can come in two kinds: positive (+) or negative (- ).
So as to move charge we need charge transporters, and that is the place our insight into nuclear particles- - explicitly electrons and protons- - proves to be useful. Electrons dependably convey a negative charge, while protons are in every case emphatically charged. Neutrons (consistent with their name) are unbiased, they have no charge. The two electrons and protons convey a similar measure of charge, only an alternate sort.
Power is quickly characterized as the stream of electric charge, yet there's such a great amount behind that basic explanation. Where do the charges originate from? How would we move them? Where do they move to? How does an electric charge cause mechanical movement or make things light up? Such huge numbers of inquiries! To start to clarify what power is we have to zoom route in, past the issue and particles, to the molecules that make up all that we associate with throughout everyday life.
This instructional exercise expands on some essential comprehension of material science, compel, vitality, particles, and [fields](http://en.wikipedia.org/wiki/Field_(physics)) specifically. We'll disregard the nuts and bolts of every one of those material science ideas, however it might counsel different sources also.
Going Atomic
To comprehend the essentials of power, we have to start by concentrating in on molecules, one of the fundamental building squares of life and matter. Iotas exist in over a hundred unique structures as substance components like hydrogen, carbon, oxygen, and copper. Iotas of numerous kinds can consolidate to make particles, which fabricate the issue we can physically observe and contact.
Iotas are modest, extending at a maximum to around 300 picometers in length (that is 3x10-10 or 0.0000000003 meters). A copper penny (on the off chance that it really were made of 100% copper) would have 3.2x1022 molecules (32,000,000,000,000,000,000,000 iotas) of copper inside it.
Indeed, even the iota isn't little enough to clarify the functions of power. We have to jump down one increasingly level and look in on the building squares of molecules: protons, neutrons, and electrons.
Building Blocks of Atoms
A molecule is worked with a mix of three particular particles: electrons, protons, and neutrons. Every molecule has a middle core, where the protons and neutrons are thickly stuffed together. Encompassing the core are a gathering of circling electrons.
An exceptionally basic particle display. It's not to scale but rather accommodating for seeing how a particle is assembled. A center core of protons and neutrons is encompassed by circling electrons.
Each molecule must have no less than one proton in it. The quantity of protons in a particle is essential, since it characterizes what compound component the iota speaks to. For instance, a molecule with only one proton is hydrogen, an iota with 29 protons is copper, and a particle with 94 protons is plutonium. This check of protons is known as the iota's nuclear number.
The proton's core accomplice, neutrons, fill a critical need; they keep the protons in the core and decide the isotope of an iota. They're not basic to our comprehension of power, so we should not stress over them for this instructional exercise.
Electrons are basic to the operations of power (see a typical subject in their names?) In its most steady, adjusted express, a molecule will have indistinguishable number of electrons from protons. As in the Bohr particle display beneath, a core with 29 protons (making it a copper iota) is encompassed by an equivalent number of electrons.
As our comprehension of particles has advanced, so too has our strategy for demonstrating them. The Bohr demonstrate is an extremely helpful molecule display as we investigate power.
The particle's electrons aren't all eternity bound to the iota. The electrons on the external circle of the molecule are called valence electrons. With enough outside power, a valence electron can escape circle of the iota and turn out to be free. Free electrons enable us to move charge, which is the thing that power is about. Talking about charge...
Streaming Charges
As we referenced toward the start of this instructional exercise, power is characterized as the stream of electric charge. Charge is a property of issue - simply like mass, volume, or thickness. It is quantifiable. Similarly as you can evaluate how much mass something has, you can quantify how much charge it has. The key idea with charge is that it can come in two kinds: positive (+) or negative (- ).
So as to move charge we need charge transporters, and that is the place our insight into nuclear particles- - explicitly electrons and protons- - proves to be useful. Electrons dependably convey a negative charge, while protons are in every case emphatically charged. Neutrons (consistent with their name) are unbiased, they have no charge. The two electrons and protons convey a similar measure of charge, only an alternate sort.


_Bohr_model.png)
0 Comments